In Short:
In late 2023, the FDA approved the first drug to potentially slow Alzheimer’s disease progression. Meanwhile, researchers at MIT are developing NeuroTrALE, a tool to simplify brain imaging analysis using machine learning. This software reduces the time needed to process vast amounts of data, aiding our understanding of brain disorders. NeuroTrALE will be open-source, allowing broader access for neuroscience research.
In late 2023, the first drug with the potential to slow the progression of Alzheimer’s disease received approval from the U.S. Federal Drug Administration (FDA). Alzheimer’s disease is among various debilitating neurological disorders that collectively impact one-eighth of the global population. While the approval of this new drug signifies progress, comprehensive understanding of such diseases remains a challenge.
“Reconstructing the intricacies of how the human brain functions on a cellular level is one of the biggest challenges in neuroscience,” states Lars Gjesteby, a technical staff member and algorithm developer from the MIT Lincoln Laboratory’s Human Health and Performance Systems Group. “High-resolution, networked brain atlases can enhance our understanding of disorders by identifying differences between healthy and diseased brains. However, progress has been impeded by inadequate tools for visualizing and processing large brain imaging datasets.”
Advancements in Brain Mapping
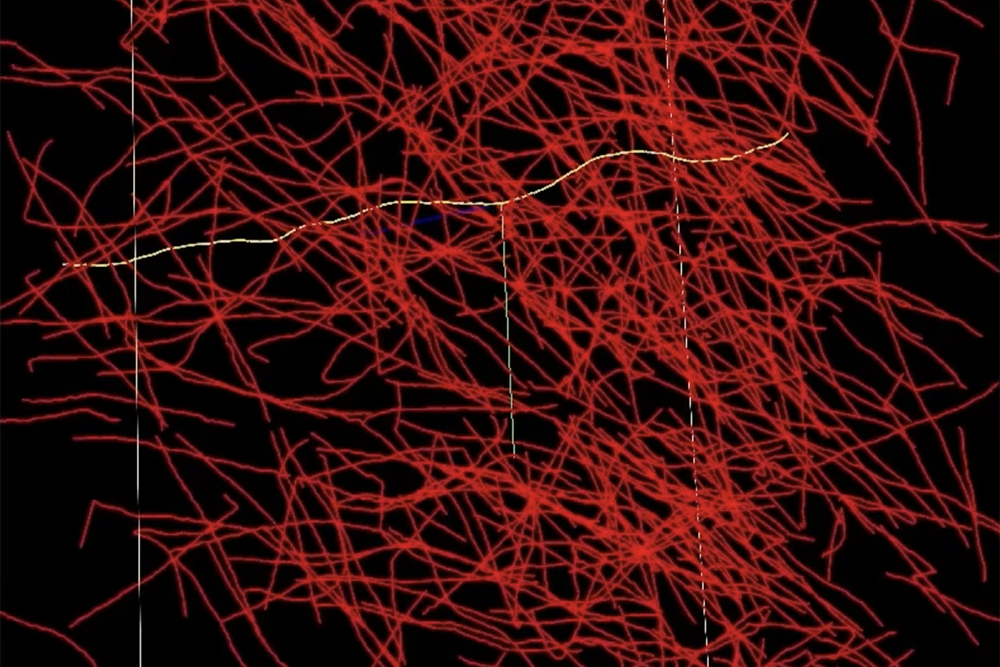
A networked brain atlas serves as a detailed map that links structural information with neural function. Creating such atlases requires the processing and annotation of brain imaging data, where each axon—thin fibers connecting neurons—needs to be traced, measured, and labeled. Unfortunately, current methods like desktop-based software or manual tools are not equipped to handle datasets at the scale of the human brain, resulting in researchers spending considerable time sifting through extensive raw data.
In response, Gjesteby is spearheading a project to develop the Neuron Tracing and Active Learning Environment (NeuroTrALE), a software pipeline that integrates machine learning and supercomputing to address this brain mapping challenge. NeuroTrALE automates much of the data processing and presents the output through an interactive interface, enabling researchers to annotate, filter, and search for specific patterns efficiently.
Active Learning for Enhanced Accuracy
One of the standout features of NeuroTrALE is its active learning approach. The software’s algorithms are designed to automatically label incoming data based on existing brain imaging information, though unfamiliar data may lead to errors. Active learning empowers users to manually correct these errors, improving the algorithm’s accuracy for future encounters. This integration of automation and manual labeling alleviates the workload on researchers.
“Imagine taking an X-ray of a ball of yarn. You’d see many overlapping lines,” explains Michael Snyder from the laboratory’s Homeland Decision Support Systems Group. “When two lines intersect, it’s uncertain whether one is bending or if they are both going in different directions. Thanks to NeuroTrALE’s active learning, users can trace these connections a couple of times and train the algorithm for future accuracy. Without NeuroTrALE, tracing the axons would require repetition each time.”
Improving Efficiency in Data Processing
By reducing the labeling effort required by users, NeuroTrALE facilitates quicker data processing. Its axon tracing algorithms utilize parallel computing to distribute tasks across multiple GPUs, significantly enhancing the speed and scalability of processing. Research conducted by the team showed a remarkable 90 percent reduction in processing time when handling 32 gigabytes of data compared to conventional AI methods.
The researchers also found that an increase in the volume of data does not directly correlate to increased processing time. For instance, a recent study illustrated that a dataset size increase of 10,000 percent resulted in only 9 to 22 percent longer processing time with two types of CPUs.
“With an estimated 86 billion neurons forming 100 trillion connections in the human brain, manually labeling all axons in a single brain would be an impossibility,” notes Benjamin Roop, an algorithm developer for the project. “This tool holds the potential to automate the creation of connectomes for not just one individual, but for many, paving the way for population-level brain disease studies.”
Open-Source Collaboration
The initiative behind NeuroTrALE originated as an internally funded collaboration between the Lincoln Laboratory and Professor Kwanghun Chung’s laboratory at MIT. The team at Lincoln Lab aimed to develop a system that would allow Chung Lab researchers to analyze and extract valuable insights from the extensive brain imaging datasets captured by the MIT SuperCloud, a supercomputer managed by Lincoln Laboratory to support MIT research. The laboratory’s strengths in high-performance computing, image processing, and artificial intelligence positioned it uniquely to overcome this challenge.
NeuroTrALE was uploaded to the SuperCloud in 2020, and by 2022, the Chung Lab had begun producing significant results. One notable study, published in Science, utilized NeuroTrALE to evaluate prefrontal cortex cell density in relation to Alzheimer’s disease, revealing that brains affected by the disease exhibited lower cell density in specific regions compared to unaffected brains. Furthermore, the same team identified regions in the brain where harmful neurofibers tend to entangle within Alzheimer’s-affected tissue.
Development on NeuroTrALE is ongoing, supported by funding from both Lincoln Laboratory and the National Institutes of Health (NIH), with efforts focused on enhancing its capabilities. Currently, NeuroTrALE’s user interface tools are being integrated with Google’s Neuroglancer, an open-source web-based viewer application for neuroscience data. This integration allows users to visualize and dynamically edit their annotated data, enabling multiple users to collaborate simultaneously on the same dataset.
“NeuroTrALE offers a platform-agnostic, end-to-end solution that can be deployed seamlessly across various environments including standalone, virtual, cloud, and high-performance computing platforms via containers,” says Adam Michaleas, a high-performance computing engineer from the laboratory’s Artificial Intelligence Technology Group. “Moreover, it enhances the user experience significantly by facilitating real-time collaboration within the neuroscience community through advanced data visualization and simultaneous content review.”
Aligned with NIH’s mission to promote shared research products, the goal of the team is to eventually make NeuroTrALE a fully open-source tool accessible to everyone. Gjesteby emphasizes that such collaborative tools are essential to achieve the ultimate goal of mapping the entire human brain for research and drug development. “It’s a grassroots endeavor where data and algorithms should be shared and made available to all.”
The codebases for axon tracing, data management, and interactive user interface of NeuroTrALE are publicly available through open-source licenses. For more information about using NeuroTrALE, please contact Lars Gjesteby.